Introduction
You can determine the equilibrium dissociation constant of an unlabelled ligand by measuring its competition for radioligand binding. This model assumes that there are two classes of sites with different affinities for the radioligand and competitor.
Step by step
Create an XY data table. Enter the logarithm of the molar concentration of the unlabeled compound into X, and binding into Y. If you have several experimental conditions, place the first into column A, the second into column B, etc. Use subcolumns to enter replicates.
From the data table, click Analyze, choose nonlinear regression, choose the panel of Competition Binding equations, and choose Two sites - Fit logIC50.
Model
logEC50Lo=log(10^logKiLo*(1+HotNM/HotKdNMLo))
logEC50Hi=log(10^logKiHi*(1+HotNM/HotKdNMHi))
Span=Top - Bottom
Part1=FractionHi*Span/(1+10^(X-LogEC50Hi) )
Part2=(1-FractionHi)*Span/(1+10^(X-LogEC50Lo) )
Y=Bottom + Part1 + Part2
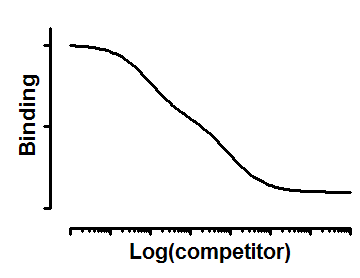
Interpret the parameters
Top and Bottom are plateaus in the units of Y axis.
FractionHi is the fraction of all the sites that have high affinity for the competitor.
logIC50Hi and logIC50Lo are the logarithms of the two IC50 values.
Notes
This model fits the two IC50 values of the unlabelled ligand. It does not report the two Ki values. The Ki values depend on the IC50s, the concentration of radioligand, and its Kd for binding. You can fit the Ki values directly using a different equation.
The analysis assumes that you know the affinity of both sites for the labeled ligand. In many cases, the radioligand has the same affinity for both sites. In that case, simply enter that value twice. If the two sites have different affinities for the labeled ligand, enter both values (determined from other experiments). Watch out for the labels. The constant HotKdnmHi is the Kd of the hot ligand for the receptors with the high affinity for the unlabeled ligand, and HotKdnmLo is the Kd of the hot ligand for the receptors with lower affinity for the unlabeled ligand. So HotKdnmHi may be larger or smaller than HotKdnmLo.
This analysis assumes that the binding is reversible and at equilibrium. It also assumes that the labeled and unlabeled ligands compete for the same binding sites.