What is an enzyme?
Living systems depend on chemical reactions which, on their own, would occur at extremely slow rates. Enzymes are catalysts that reduce the needed activation energy so these reactions proceed at rates that are useful to the cell. The study of enzyme kinetics can help us understand the function and regulation of enzymes.
Enzyme progress curves
In most cases, an enzyme converts one chemical (the substrate) into another (the product). A graph of product concentration vs. time follows three phases marked on the graph below.
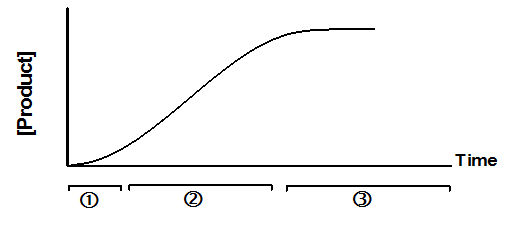
1.At very early time points (usually less than a second), the rate of product accumulation increases over time. Special techniques, not available in Prism, are needed to study the early kinetics of enzyme action. The graph above exaggerates this first phase.
2.For an extended period of time, the product concentration increases linearly with time. All the analyses built-in to Prism use data collected during this second phase.
3.At later times, the substrate is depleted, so the curve starts to level off. Eventually the concentration of product reaches a plateau.
It is very difficult to fit a curve to these kind of data. The model simply cannot be reduced to an equation that expresses product concentration as a function of time. To fit these kind of data (called an enzyme progress curve) you need to use a program that can fit data to a model defined by differential equations or by an implicit equation. For more details, see RG Duggleby, Analysis of Enzyme Reaction Progress Curves by Nonlinear Regression, Methods in Enzymology, 249: 61-60, 1995.
Rather than fit the enzyme progress curve, most analyses of enzyme kinetics (including all those built-in to Prism) measure product at a single time point. Analyses assume that the time point you chose is on the linear (second) phase of product accumulation and ignore the nonlinear first phase (which is usually very short). Therefore, if you divide the amount of product produced by the time the reaction was allowed to proceed, you compute the amount of product formed per unit time, which is the enzyme velocity.
Terminology
The terminology can be confusing. Note these confusing points:
•As mentioned above, almost all studies of enzyme "kinetics" are done by collecting data at a single time point. The X axis is substrate (or inhibitor) concentration, not time.
•The second phase shown in the graph above is often called the "initial rate", a phrase that makes sense only if you ignore the short transient phase that precedes it.
•That second phase is also called "steady state", because the concentration of enzyme-substrate complex doesn't change during that phase. However, the concentration of product accumulates, so the system is not truly at steady state until, much later, the concentration of product truly doesn't change over time.