I entered "dilution factors" into the X column of my data table. Can Prism convert these to concentrations?
If your dilution factor values take into account all serial dilution steps and can be related to a single stock concentration, then this is easy to do using a "User-defined X transform". Suppose you diluted a 1 mM stock solution serially in three 10-fold steps. You then assayed aliquots from the resulting solutions, diluting another 5-fold in the process of addition to each reaction tube. Enter the total dilution factors into the X column, and your assay results in the Y column(s).
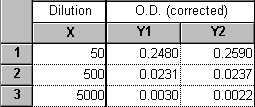
Do a transform ("Analyze...Data manipulations...Transforms") on the X column. Choose "User-defined X transforms" in the "Parameters: Transforms" dialog. Name your transform, choose "Add", and enter the transform X=K/X (K represents the millimolar concentration of your stock solution).
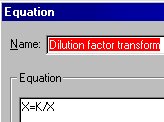
When you exit this dialog, you'll see a box for the value of K. For this example, you would enter 0.001, for 1 mM. Click "OK" to do the transform.

The Results sheet now shows the final molar concentration of your reagent in the X column (if you are using a column heading, you may wish to update it as shown here).

Two further notes:
- Instead of a user-defined transform, you may wish to chain two built-in transforms together, X=1/X and X=K*X.
- If your dilution scheme is more complicated, you might need to do the computations on an embedded Excel spreadsheet (Prism 3.00 or later). See the chapter "Importing and Pasting Data" in the Prism 3 manual for details on integrating a spreadsheet into Prism.















